It has been shown that an absorption test according to the method I have developed, not are entirely reliable. Under normal circumstances should it work, but if the fuel contains substances that lower or increase the fuel's water-absorption capacity, is the test not accurate.
At present it is not known what substances which affect the water-absorption for E85, so to further may one assumes that the fuel is acceptable if its density is close to the normalized values. If the density is found to be normal so can the absorption capacity in fact be lower or higher than a strict mix of ethanol and gasoline can receive. In this case, pointing it at that the fuel contains an unknown component that only affects the absorption capacity - with a density comparable to pure ethanol. Are the density higher without that the absorption capacity shows an increased level of water; then would the fuel be able to contain a salt or a substance that dissolves in gasoline or ethanol. Is it a substance or a medium, then should its density be close to the density of water.
You can not get a reliable density value by measuring the density of intact E85, because the density of the gasoline component can vary quite widely. One must first distinguish the gasoline, so that the sample only contains water and alcohol. By adding water in E85 stratifies the gasoline component, but the water-soluble components can not be avoided. Such an element is ether-oxygenates or MTBE. The content of the ether-components can vary but for normal E85 are they quite stable in quantity.
The easiest way seems to be to use an hydrometer to measure the alcohol content. Such an analyzer could I get at City Gross. Hydro meters are usually graded in volume (tralles).
One can start the measurement procedure by first determining the distribution of gasoline and ethanol. It is important to know how much gasoline it is, otherwise one not know the proportion of ethanol, either. When the proportion of gasoline is defined can the water concentration in the ethanol component be calculated, but if the density for the sample is less than pure ethanol - must it contain something that is lighter than ethanol. The most likely scenario is that it then contains an excess of ether-oxygenates.
Start by making a quantity test:
- Measure 50 ml E85.
- Measure 48 ml water (preferably dosing saline in the water so the separation of water/gasoline becomes more distinct).
- Mix them, seal and shake for 15 seconds. A graduated cylinder for a hydrometer is a suitable containers.
- Release the pressure, but seal the container again and let this layer in 15 minutes.
- Read the gasoline volume in milliliters after 15 minutes.
- Insert the volume into the formula: weight per cent of ethanol = 100 - (2.1 + 1.94 x gasoline volume)
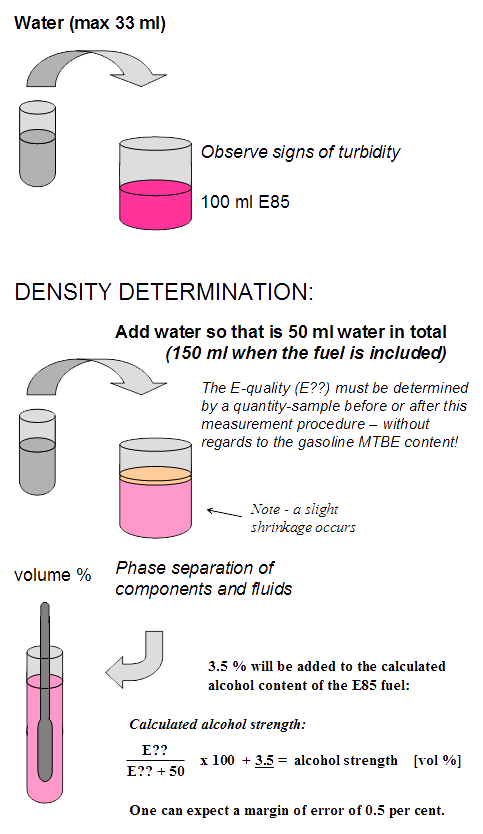
Perform an absorption test before the density test by gradually adding water to 100 ml E85. Once you finished, can you determine the quantity of any water with this Excel program. Then add additional water so the total volume reaches 50 ml of water.
Let this mix separating in peace a few hours - then, can the measurement of density be made with an hydrometer in the alcohol part. Then use this Excel program to determine water content.
When the measurement process is complete, there are two results that can either work together or contradict each other. So... if both of the tests showing that the fuel contains water is it of course more likely that it really contain water - than if only one of the samples would show it. This, together with a test of the electrical conductivity, will further give support to this conclusion.
An illustrated version follows below
It is best to first make an absorption test:
This analysis can be downloaded here.
|
| |
|
| |

