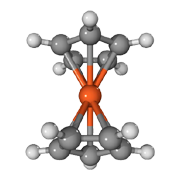
Ferrocene C5H5-Fe-C5H5
Benzene ring C6H6
Pentadiene ring C5H6
Ferrocene is therefore an iron metal salt of cyclopentadiene acid. Compared for example iron benzoate (i¤b) which is an iron metal salt of benzoic acid. Conversely dissolves iron benzoate best in alcohol and not so good in gasoline, while ferrocene dissolves easily in gasoline, benzene, toluene and acetone but pretty bad in alcohols. Even diesel and white spirit is good solvent for ferrocene.

Often one can mix the purchased powder directly into gasoline without any insoluble impurities are left unsolved. If one in the other hand wants to solve ferrocene in diesel or alcohol so should one be aware that not all will be solved which mean that the fuel filter after some time can become clogged. Separation or filtration may therefore be necessary in some cases. The color of the fuel is changed at the red direction and where the combustion takes place forms a reddish coating of iron oxide. Ferrocene can probably be regarded as the main ingredient in so-called MPG-Caps, or colloquially: fuel tablets. After numerous tests, I note that ferrocene together with hexamine (HMTA) have no or little importance to achieve lower fuel consumption, apart from this increases ferrocene the anti-knock ability and contribute to a less soot.
Ferrocene dissolves also good in nonpolar fuel additives and its possible to dissolve 5 ml of ferrocene in 100 ml A40 (at most). Other additives that could keep ferrocene is: ISO, X66, AT2, hoGA etc. The fuel additive A40 can also be prepared with omega-Fe without that the solubility of ferrocene decreases significantly.
The big disadvantage of ferrocene is that the iron trioxide layer conducts current. There are two oxide types formed: one is
iron trioxide and the other is iron tetraoxide. Tetraoxide is not conducting which however trioxide is. If the trioxide dominates around the spark plug will it
leads to a short circuit - instead of an arc flashover. This has the consequence that the spark fails - the engine misfires. You can tell it easily and it can
be fixed by cleaning the plugs. It's therefore not a clever idea to dispense too much ferrocene and one should be fairly cautious with ferrocene whatsoever. The
only benefit of ferrocene as I see it is to mix it with diesel, where no spark plugs can be found.

My snip I did on eBay - 250 grams for $20. Only one distributor could be found but the price was affordable. 5 ml weighs about 2.5 grams. The most costly component is however HMTA - unless you can get it cheap. Then acetone which now is quite expensive in Sweden. Perhaps we should produce HMTA and acetone oneself? There are certainly pages about this online or on Youtube.
|
| |
|
| |




