This oil is a sacred cow as someone said - for there are many opinions here. To make it short; it dealing about which one is best - mineral or synthetic? But the whole subject is controversial - it tends to be so when the question is vague or difficult to grasp. Mineral oil is good when you run in a motor, because it allows the piston rings and the liner to touching each other - so that the engine is running in more efficient. A synthetic motor oil separates the metal parts and are best when the engine is ready and throughout its continuing life.
People often knows that mineral oil is made from a distillation from crude oil (known as refining), but that synthetic oil also is originates from a refined petroleum base, is perhaps not obvious to everyone? Exactly how it all works, I dare not to explain but everything seems to be about various more or less complicated refining processes. For this reason parts the oil in groups, where 1-3 is derived from an ordinary refining, ie production of mineral oil, and where 4 and 5 is synthetic oils. The snag is that the oils in group 2 and 3 can use the word ”synthetic” to mislead not hurt guessed consumers, as synthetic-based, synthetic formula, chemically altered, etc. Instead of calling it half synthetic and fully synthetic. However, up to 15 % of the engine oil consisting of different additives.
This I found online:
1. Pour point depressors
2. Viscosity improvers
3. Additives that form tribological films
4. Friction modifier (FM)
5. Abrasion Reduction additive (anti-wear)
6. Extreme Pressure additive (EP)
7. Cutting and welding-limiter
8. Additives prolong oil's life
8.1 Defoamers
8.2 Dispegents
8.3 Detergents
8.4 Antioxidants
8.5 Passive Rare Metal
9. Corrosion inhibitors
2. Viscosity improvers
3. Additives that form tribological films
4. Friction modifier (FM)
5. Abrasion Reduction additive (anti-wear)
6. Extreme Pressure additive (EP)
7. Cutting and welding-limiter
8. Additives prolong oil's life
8.1 Defoamers
8.2 Dispegents
8.3 Detergents
8.4 Antioxidants
8.5 Passive Rare Metal
9. Corrosion inhibitors
The most significant seems to be:
(2) Viscosity improvers - about 25 % the viscosity is in check at higher temperatures. Names like polymetacrylate, styrene-isoprene.
(8.1-5) Dispersants/detergents - about 50 % prevents oxidation, rust, corrosion on non-metals, foam formation, keep dirt suspended in the oil, keep the engine clean, cold to warm, the list is long. Named as; sulphonates, phenats, sucinamids, (Metal bases, organic substances), etc.
(1) Pour Point depressors - prevents the paraffin oil to crystallize in the winter.
(5) 10 % of the package prevents metal-metal contact, such as molybdenum disulphide.
(8.1-5) Dispersants/detergents - about 50 % prevents oxidation, rust, corrosion on non-metals, foam formation, keep dirt suspended in the oil, keep the engine clean, cold to warm, the list is long. Named as; sulphonates, phenats, sucinamids, (Metal bases, organic substances), etc.
(1) Pour Point depressors - prevents the paraffin oil to crystallize in the winter.
(5) 10 % of the package prevents metal-metal contact, such as molybdenum disulphide.
I let photograph three interesting factory owner of engine oil (the oil is new and unused):


1. Biltema ”Synthetic” 5W-50, price 69 SEK.
2. Goth Oil (Mineral Oil) 10W-40, price 35 SEK.
3. Havoline ”Party Synthetic” 10W-40, price 59 SEK.
Rates current 2010-07.
The difference in color is nil. The color is quite dark but when you use eg one test-tube is the color light brown because the light penetrates more. I was informed that, if you mix synthetic motor oil with mineral oil can they layering itself. I tested it in a test-tube and it is possible to see, that the two oil types grudgingly allow themselves to be mixed together. The smell is different, however, when Goth Oil smells a bit like a perfumed old oil pit, with a hint of burnt - while the synthetic oil from Biltema smells sophisticated. Subsequent information (where the source is an engine knowledgeable employee at a car company) claiming that most engine oil brands, such as Biltema's oils, Havoline and those who is available to get from gas stations etc. - is the same thing, thus as coming from the same grosist; namely Statoil, except Goth Oil which comes from Turkey.
Further down, I will describe a very simple method of purification for used motor oil.
Under normal circumstances can the motor oil be purified and then used as if it were new. That who happens in a car engine is that the oil quickly becomes second-hand because it get dirty with soot, metal salts and metal particles. Can you keep after this problem so the life is extended, but not very extended! The additives in the oil will be consumed and it is suited to the change intervals - but with a certain overtime reserve, however.
After what I have seen is the oil after about 6000 miles with E85 in better condition than that of gasoline - because there is less soot. But I think old motor oil as a whole, still seems to have left their good qualities. The smell is not particularly sharp (petrol), which also seems to be the same viscosity as new oil. Aside from the color, it is difficult to see with the naked eye any difference in comparison with new oil. When I gradually warmed up the waste oil to 450 degrees Fahrenheit, I could barely see a few trends to decoction of volatile compounds (of water, gasoline, ethanol, etc.), even though it contained my own teflon-additive! In fact, it never began to boil, however, oil began to steam at about 340 degrees and steam rose after each temperature step - ie the lighter petroleum based (oil components) withdrew away. The old and used engine oil (which is from my car) was therefore in principle free of both water, ethanol, gasoline and antifreeze (glycol)!
When I purposely added glycol, water and ethanol in the oil, it seemed clear that it departed from the moment it was approaching 210 degrees, then boiled the oil up to 280 degrees and the brown coating/foam withdrew away which meant that the oil adopted the black color again. At about 350 degrees began it boiling again and as I understand, it was the glycol with a small component of Teflon. In a later attempt, I have found that Teflon's boiling point is located at 340 degrees, but I have also found that pure glycol has a boiling-range (in engine oil) between 280-390 degrees. As I understand it, a warming of engine oil shall not exceed 390 degrees, but if you choose to distil the oil in a container so can you probably assume that something has happened at 390 degrees, on the other hand, I had no indication at this, during the warming? But the oil smells different when you warmed it over 250 degrees - a bit burnt as well. Perhaps even the additives are harmed by such temperatures? In a car engine, the oil is sprayed around and moving very much and, therefore will also the evaporation of such as glycol be easier although the temperature is relatively low.
The refined petroleum component, ie the lubricating-oil itself, will not be consumed/deteriorate (unless it is burned). This can not wear out but it can be contaminated by gasoline, ethanol, water, glycol, metallic particles and soot particles. While the additives are stop working, can the internal parts of the engine corrode, wax can be activated (in cold weather) and increased wear may be due metal-metal contact. In fact can the vapors through the vented crankcase take away water, gasoline, ethanol and glycol - if it worked properly and I think it does so, on my car at least? Water, gasoline, ethanol (and perhaps a bit glycol) are temporary guests who keep coming on and it is important to throw them out as soon as possible. Functioning crankcase ventilation is the alpha and omega if you want an oil with some good qualities. Glycol in the engine oil is directly harmful as it significantly increases wear! Would you listen to reason on this issue, it should at least older cars get their oil assayed at regular intervals, in order not to risk a breakdown, especially in view of the glycol then... For instance, it could include one (free) analysis, at the annual inspection.
Continuing, we have soot, but above all the metal particles, but this should not be a problem either. An oil filter most important task is to clean the oil from the metal particles and this issue can most of this filters manage splendidly - until it is clogged with metal dust... So, what does this ultimately? As I see it, works the engine of the car fine as it is, but if it's running with a additive containing glycol should the crankcase ventilation be extra efficient, so that no hazardous quantities is collected in the engine oil. - What happens then? - Friction/wear increases.
You can add Teflon if the friction increase - but follow my recommendations so you do not add too much! An increase of glycol in the oil should be tackled with a Teflon additive, because the Teflon will compensate the harmful effects of glycol. Then an effective vented crankcase are constantly keeps the pollutants at bay, so there is only one fear still and it is the oil filter. - What should I do to prolong the life of an oil filter? - Is there any idea to remove the engine oil and clean it sometimes, for to extend the oil filters life? - Not really, when the oil contains no harmful metal particles (it is located in the filter) so will you not find them in the oil, unless the oil filter is clogged - but the risk is low.
Subsequent information claiming that it is precisely the small metal particles that cause the most trouble - especially when it comes to oil for hydraulic systems. According to Hydro Swede causing the increasing amount of micro particles greater wear and also to the oxidation of the oil. This is because that the oxidation is catalyzed by small metal particles. The amount of micro particles increases when the engine gradually grind down larger particles and besides will the micro particles pressed into the engine material, this because that the contact area is small.
Hydro Swede has categorized three types of pollution:
- Hard contaminants (metal, sand, dust particles, fibres, etc..)
- Soft contaminants (oxidation products from the oil and reagent products from the additives)
- Water (free or emulsified water)
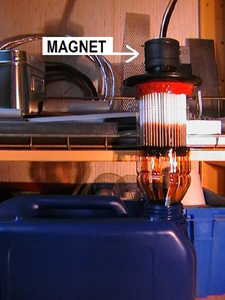
Metal dust is also composed of aluminum - which not is magnetic, but if you puts a number of neodymium magnets on the oil filter casing so should iron dust accumulates at these instead of getting stuck in the filter paper. The oil filter life (in this case) increases then, because the magnets are attracting both large and small particles. If you have an extra filter, eg a COT-filter or a centrifuge, so of course this relieves the regular filter and the oil purification becomes more efficient - ie the life of the oil filter is increasing. Some information about COT.


The left image shows an oil filter with 12 strategically placed neodymium magnets on its outer surface. You do not have to attach the magnets, because they attach themselves, when the filter housing (for this filter) is of sheet steel. - What happens if the filter becomes hot? - Nothing, the magnetism is permanent even if it lowers them into boiling water. Placing the magnets in this way make sure that the filter ability increases significantly. One could also add a magnet on the drain plug - if there is not already one there?
The middle image shows oil's path through the oil filter and the rubber stopper that works like a check valve, so as not dirty oil drain back again but also as a dust barrier. The picture at right is the filter element and the bypass valve, which is a click type.
Oil filter housing consists of 0.7 mm sheet steel and unfortunately it is enough to reduce the magnetic force of a small neodymium magnet - where metal particles are trapped poorly or not at all. Get more larger neodymium magnets, or place seven pieces of magnets (as shown) together, over each other - because then the force will be focused and probably depends it on the ring shape. Ring-shaped magnetic allows the magnetic field to sweep across all (up to seven) magnets like a coil magnet. Clas Ohlson also sold cube neodymium magnets (which has now been discontinued), that provide the same effect as seven of the others did - but these get not stronger if you pack them.
So what is the case if you get glycol into engine oil? As I said earlier, increasing friction and engine parts wear out but this can be compensated with Teflon. What also happens is that the oil viscosity increases, at least if you believe some horror stories circulating on the net? What I have found is that of adding salts of various kinds (in engine oil) increase the viscosity - it is a fact. Then the cheap ethylene glycols containing salt-based inhibitors, should it mean an increase in viscosity? Now it so, I have mixed different varieties glycol in oil and also added both water and ethanol but the increases of the viscosity/lump trends could not be observed, although there were large amounts and then heated to 250 degrees. I also mixed inexpensive ethylene glycol with more expensive (G30) without any sort of reaction started (even heated and with water). Instead, the mixture became purple and then the red was mixed with the blue. The same was valid to propylene glycol; for how I than mixed this type of glycol with the others - nothing happened, except the color shifting. It is therefore noted that the glycol varieties that are sold are pretty forgiving, because you may well expect that there are quite a lot of blockheads out there and one small mistake may not lead to a disaster - it is the thought. But a gasket can get broken and it would indeed be sad if the whole engine cut away for it? Would you like to have ethylene glycol with no additives at all, so you can distill it. I've practiced and it is a simple process. After distillation of the cheap ethylene glycol (containing salt inhibitors) should it be in principle completely clean afterwards.
For us amateurs, it feels somewhat overly ambitious to build a centrifuge or to install a self-designed COT filters on their car. You could drain the oil and let it simmer in a large saucepan, and then fill it again into the engine - through a filter? Such a filter, for example, would be able to be constructed of a small plastic box, filled with cotton pads or why not with a HEPA filter (see below) and with cotton pads in it? But as I stated earlier there is already an oil filter, though it might not be so effective?
No, I advocate the method with neodymium magnets and the HEPA method as a complement. By recycling their old motor oil so can you use it again. But when the additives are almost battered, you can instead mix it up with an equally large amount of new motor oil. At that way you only need to buy half as much oil when it's time for an oil change. But in the meantime the oil is used so can you fill in (when necessary) with the part left over.
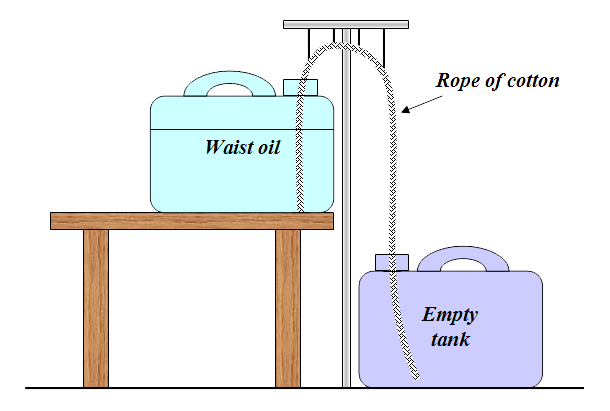
By capillarity effects, the oil hike in the rope from the upper to the lower oil drum. Once the oil is approaching the empty container began the gravity to draw the oil like the siphon principle. This causes the waste oil transported automatically between the drums. All particles remain in the rope which then may be thrown. In the past, they fired up the rope but this method can probably be called into question today? The purification process takes time! One should expect a few weeks before it is finished. The whole thing should be encapsulated during this time; otherwise the oil is mixing with the dust in the air. Cotton rope can be difficult to find but Ropes Direct sells thin cotton ropes you could entwine. The rope diameter should be about half an inch.



After an attempt with the help of two test tubes and a cotton rope (which I suggested before), can it be seen that soot particles do not stay on the rope but is supplied with the oil, throughout the whole trip. Since soot particles comes with is the question, if something else also comes with? The fact is that the oil is much cleaner since the passed oil smells much better - as if it were new.
Jula also sells HEPA-filters (high efficiency particulate air) for vacuum cleaners who also is washable. A HEPA filter (which is an air filter) does not let through larger particles than 0.3 microns and that is far below what is allowed to pass an ordinary oil filter, which has its limit around 40 microns - in other words it diffs 130 times between the filters! There are different classes of HEPA filters: H10, H11, H12, H13 and H14. H10 stops 85%, while the H14 in principle filter out everything. A vacuum cleaner is usually provided with two HEPA filters, one coarse and one fine. Remember that the class rating takes into account a strong air flow - not a slow flow of a fluid.
Biltema have an HEPA filter, art 84-0680 (has now expired from the range).Probably a H10? I bought such a filter and managed to push a cutted plastic bottle of white spirit into it, which I later glued with Biltema's high-temperature silicone. I did not need to cut or file down any. It fitted pretty well, though it was a bit tight. The only thing I did was to roughen the surface where the silicon would draw. It was a fairly simple design - as shown below. The image is upside down as this should be the direction of the oil flow, but it can be discussed? In order to get some pressure on the oil so can you connect a link to the filter unit and a hose on to a can a few feet above the filter. The filter can be cleaned by allowing new oil (Goth Oil mixed with white spirit) to flow in the opposite direction. Place readily a magnet in the oil route, so you get some idea of engine condition.


 All the grime will not go away!
All the grime will not go away!
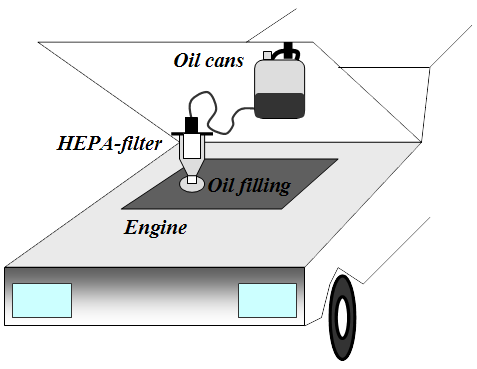
A HEPA purification of the engine oil should mean that the oil's life span is increasing, like COT-filter system. One should therefore be able to mimic such practices, by to one or two times perform a purification process of the engine oil. The whole thing is quite simple both in its design and in its execution. You thus acquire a HEPA filter and a can, then to makes the necessary modifications. In addition, we must purchase a pump for engine oil and such thing has Jula in its range.
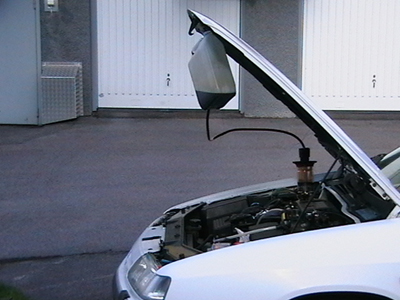
Run the engine warm, then connect the filter over the engine oil filler plug and adapt the can. Pump up the oil from the engine to the can with a hand or electric pump. When all the oil is transported to the can, lift it up and hang it under the bonnet. Then, just wait until the oil has flowed back again.
If one pumps up oil from the sump when the car has been standing still for a longer period (cold engine), so is there a chance that it sucks up sludge, water, glycol, etc. The actual cleaning process may instead be performed indoors where it's warmer - but the worst (first) oil should be discarded immediately. To bring this process to proceed more quickly when one are indoors can the distance between the filter and the can, be extended.


To increase the credibility of what I say have I myself availed the method. The hand pump worked great and with this I first sucked up cold oil, which have been laying about one day, however seemed no mud exist or anything else unusual. I was using a Festis bottle for this. Then I drove the engine warm and filled the can with oil. Only half of the can was filled, so I did the cleaning process twice after I started the engine in between. It proceeded as I had thought - a relatively simple operation which in its design reminiscent of dialysis, when you equate blood with motor oil.


|
| |
|
| |

